Edit Content
Trending
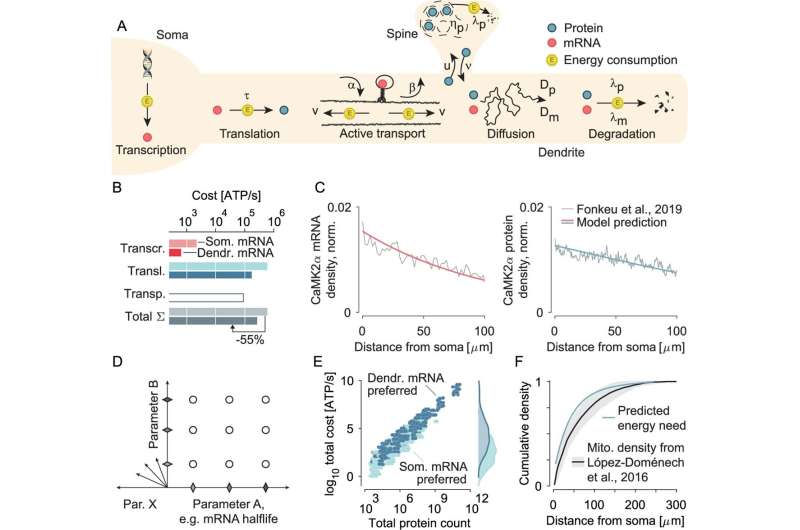


Nerve cells have amazing strategies to save energy and still perform the most important of their tasks. Researchers from the University Hospital Bonn (UKB) and the University of Bonn as well as the University Medical Center Göttingen found that the neuronal energy conservation program determines the location and number of messenger RNA (mRNA) and proteins and differs depending on the length, longevity and other properties of the respective molecule. The work has now been published in Nature Communications.
We have all experienced the need to save energy in recent years. To do this, we all had to come up with strategies to save energy while still meeting our most important needs.
“Our nerve cells are facing a similar dilemma: They have to supply their synapses, i.e., their contact points with other neurons, but also organize their protein synthesis in such a way that they don’t produce too much or too little proteins.
“At the same time, they have to transport the proteins over long distances to the synapses and also pay attention to their energy budget. How do they manage this?” asked the research team.
Despite its relatively small size, the brain consumes about 20% of the body’s total energy. As with all cells, neuronal functions are subject to strict energy constraints, which are particularly pronounced in the brain due to its high energy requirements. The research team was able to show that the synthesis and degradation of all neuronal molecules represents a particularly high cellular energy expenditure and therefore requires energy-saving measures.
All cells, including neurons, require proteins to function properly. These are produced through a process known as gene expression, in which the relevant information is copied from a gene into messenger RNA (mRNA). The mature mRNA is then translated into the required protein.
Thanks to advances in biochemistry and microscopy, it is now possible to precisely map the location of individual mRNA copies and the corresponding proteins in cells and quantify the number of thousands of mRNA and protein species. For the first time, this enables researchers to study complex organizational principles that control spatial gene expression patterns and apply them across all types of molecules.
The research team, led by corresponding author Prof. Prof. Tatjana Tchumatchenko, research group leader at the Institute for Experimental Epileptology and Cognition Research at the UKB, combined experimental data from more than 10 large-scale mRNA and proteomics screens involving tens of thousands of molecular species.
“We found that the drive to conserve energy determines mRNA and protein number and location, affecting each molecular species differently depending on the length, lifetime and other properties of the molecule,” explains first author Cornelius Bergmann, a Ph.D. student of the University Bonn at the Institute of Experimental Epileptology and Cognition Research at the UKB.
The results show that the energetic costs for synthesis, transport and degradation of molecules, their spatial localization and the total number are limited to energy-efficient solutions. “If certain short-lived proteins were synthesized in the cell body, a large proportion of them would not arrive alive at the synapses due to the long travel time,” adds Prof. Tchumatchenko. “This would be a waste of energy on proteins that cannot fulfill their task.”
The model calculations in the present study show that proteins are therefore preferably synthesized in the branched, tapering extensions of a nerve cell, the so-called dendrites, if the energy loss “on the way” from the cell body to the synapses is greater than the energy required to transport the mRNA into the dendrites.
Discover the latest in science, tech, and space with over 100,000 subscribers who rely on Phys.org for daily insights.
Sign up for our free newsletter and get updates on breakthroughs,
innovations, and research that matter—daily or weekly.
However, the research team’s findings go beyond energy conservation. “Our results shed light on the organizational principles of gene expression in cells that act across different molecular species and go beyond individual regulatory mechanisms,” says co-author Prof. Silvio Rizzoli, Director of the Department of Neuro- and Sensory Physiology at the University Medical Center Göttingen, spokesperson of the Center for Biostructural Imaging of Neurodegeneration (BIN).
The most surprising result for the research team was that the physical properties of proteins, such as length or lifetime, and not their specific function, have such a strong influence on the energy budget and thus on the site of their synthesis.
Co-author Kanaan Mousaei, a doctoral student of the University Bonn at the Institute of Experimental Epileptology and Cognition Research at the UKB, emphasizes, “Our model offers a new perspective for correlating dozens of existing data sets from different laboratories.”
More information:
Cornelius Bergmann et al, How energy determines spatial localisation and copy number of molecules in neurons, Nature Communications (2025). DOI: 10.1038/s41467-025-56640-0
Provided by
University Hospital of Bonn
Citation:
Nerve cells optimize energy by controlling mRNA and protein distribution, study finds (2025, February 7)
retrieved 7 February 2025
from https://phys.org/news/2025-02-nerve-cells-optimize-energy-mrna.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.
©2024. Livebuzznews. All Rights Reserved.